Development of a Method for Winter Road Surface Condition Prediction for Wider Regions
Background of the study
In snowy and cold areas, snowfalls and road surface freezing in winter greatly affect road traffic. Therefore, to secure safe and smooth road traffic, winter road management activities such as snow removal and spreading anti-icing agents have been conducted. Because of nation-wide issues, including the declining population, demographic aging, and financial constraints, efficient and effective operations of winter road management have been called for.

by a measurement vehicle
(Right: The infrared thermometer is installed in
front of the radiator directing downward.)
temperature and conditions are predicted in
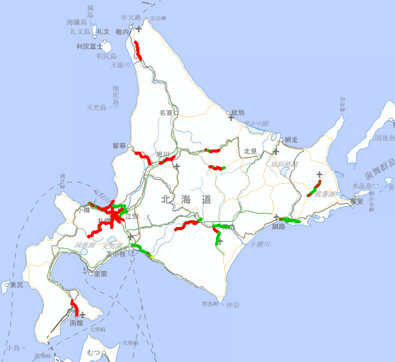
Hokkaido (Red and green solid lines)
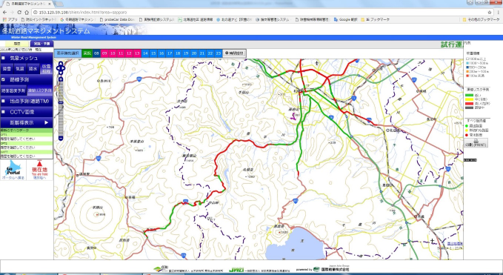
results by the Winter Road Surface Management
Support System
Overview of the research results
Traffic Engineering Research Team has been contributing to efficient winter management for the national roads in Hokkaido. For the road administrators, we provide the Winter Road Surface Management Support System that predicts the surface temperature and other conditions of winter roads and a simulator that supports winter road surface management by predicting the efficacy of spreading anti-icing agents. The Winter Road Surface Management Support System uses thermal mapping data (i.e., measured values of the longitudinal distribution of road surface temperatures) and weather data. For collecting the thermal mapping data, it is necessary to run vehicles with on-vehicle temperature measurement equipment (Fig. 1), which requires high costs and a great amount of labor. Thus, the support system has predicted about 600km in a total of about 6,700km of national roads in Hokkaido (Fig. 2). The time required for calculation using the system has exceeded one hour, which has been an issue to be solved. To solve these problems, we started to use assumed thermal mapping data that was created by using an air temperature-road surface temperature equation and weather mesh data. We also improved the calculation speed by developing a technique in which the number of calculation points was reduced for sections with a relatively uniform road environment and linearly interpolated the data for the reduced points (i.e., a technique to calculate the approximate values between two known data points by assuming the values distribute linearly). By using this technique, we were able to achieve a prediction accuracy that was equivalent to that obtained by using the conventional technique and to shorten the calculation time to about 10% of the conventional technique. In the winter of 2019-2020, we incorporated this newly developed technique into the Winter Road Surface Support System and increased the road length covered by the road condition prediction by about 100km (Fig. 3). We will continue to increase the road length covered by the assumed thermal mapping data and improve the road condition information provision.
(Contact: Traffic Engineering Research Team, CERI)
Web Magazine of the Public Works Research Institute, Vol. 63 (Introduction of Research) Efforts to realize easy judgment for acid sulfate soil
samples after mixing a hydrogen peroxide
solution of approx. 3%
after mixing a hydrogen peroxide
solution of approx. 3%
Acid sulfate soil (ASS) contains sulfur compounds, which easily oxidize and becomes sulfate. Therefore, ASS is already strongly acidic or becomes strongly acidic with the oxidation progress when it comes in contact with air. When ASS is immersed in water and in a reductive state (oxygen is deficient), the soil is nearly neutral, which causes no problem. However, when the water around ASS is drained by digging, and the ASS is exposed to air (oxygen) and oxidizes, the oxidized ASS generates a large amount of sulfate that drastically lowers the pH of the soil. The soil becomes strongly acidic. Such acidic soil is troublesome because plants cannot live under such acidic conditions, and metals and concrete will quickly corrode.
ASS is generated in the sedimentation of organic soil in a reductive condition, such as in seawater containing sulfuric acid ions. Hydrogen sulfide is generated when organic soil contacts seawater, reacts with divalent iron in soil, and generates iron sulfide minerals, which are sulfur compounds. Because of this generation process of ASS, ASS is often found in lowlands near coasts or areas near river mouths that are influenced by seawater. ASS also unevenly distributes in the soil layers generated as a result of sea bottom uplifting or those generated during volcanic activities.
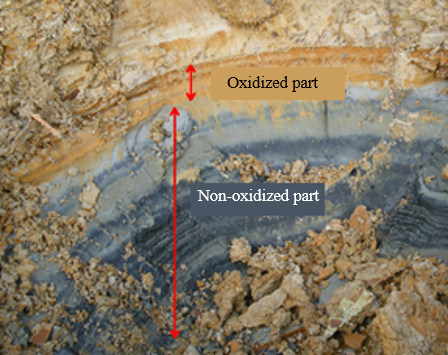
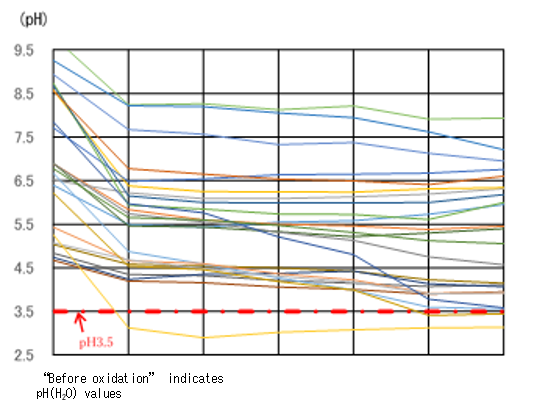
The color of ASS is from bluish-black to bluish-gray in the reductive condition and reddish-brown to pale grayish-yellow in the oxidized state (Photo 1). The shear surface of the ASS block sometimes gives off odor similar to that of rotten eggs. In most cases, ASS is odorless. Therefore, it is difficult to determine on the spot if the soil is ASS when bluish-gray soil is found. Weathered mudstone or other soil in the reduced state has a similar bluish-gray color. Determination of ASS is done by oxidizing the sample with a hydrogen peroxide solution (H2O2 of approx. 30%) and measuring the pH value. A hydrogen peroxide solution of approx. 30% is hard to obtain and handle because it is designated as a deleterious substance by the Poisonous and Deleterious Substances Control Law. Therefore, when it is possible that soil found at a construction site is ASS, the soil sample is sent to a specialized institution for analysis and determination. The construction of that site has to be suspended for one to two weeks before the determination is done by the institution.
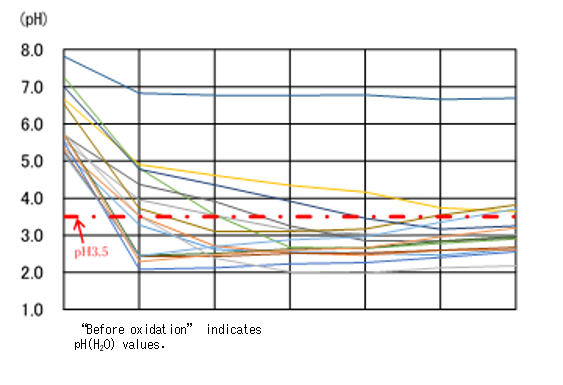
The Rural Resource Conservation Research Team has been conducting research to develop a simple ASS analysis technique that can be used on-site to determine ASS in several hours. To oxidize soil samples using this technique, instead of a hydrogen peroxide solution of approx. 30%, we used a hydrogen peroxide solution of approx. 3%, which is easy to buy and handle. At a room temperature of 20℃, we mixed soil samples with the hydrogen peroxide solution of approx. 3% at a ratio of 1:5, agitated the mixture, and left it at rest. In 6 hours, 13 samples among the total of 15 non-oxidized soil samples exhibited pH values of 3.5 or lower (Fig. 1). The threshold pH for determining ASS is 3.5. The reaction in one sample differed from the others. That one sample contained a large amount of manganese that dissolves H2O2 into water and oxygen while foaming actively. There was one sample whose pH did not become lower than 3.5 because of its slow reaction. Among 24 non-ASS samples, one maintained low pH values throughout the test (Fig. 2). Our team continues research for developing a quick and easy-to-use ASS determination technique, including analyses on soil that shows reactions which differ from the majority of the others, such as stated above.
(Contact: Rural Resource Conservation Research Team, CERI)
